At present, the trend of using plant proteins and polyphenols as functional food components is becoming more and more obvious. Therefore, in this study, colloidal complexes were prepared using pea protein (PP) and grape seed proanthocyanidins (GSP) as raw materials, and their formation and stability were investigated. The ability of oil-in-water emulsions. The main interaction between PP and GSP is hydrogen bonding. The stability of PP-GSP complexes to environmental changes is as follows: pH (2-9); ionic strength (0-0.3 M); temperature (30-90 °C). The emulsion prepared with PP-GSP complex as emulsifier has a smaller average particle size (~200 nm) and a strong negative surface potential (~-60mv). Compared with PP alone, the PP-GSP complex can slightly reduce the isoelectric point, thermal stability and salt stability of the emulsion, but the storage stability of the emulsion has a certain improvement effect. The presence of GSP gives the emulsion a deep orange-red (red-yellow) color, which can be used for some specific applications. The findings contribute to the efficient delivery of proanthocyanidins into food matrices.
Pea protein is a surface-active protein that can be used as a natural emulsifier for the preparation of emulsion-based delivery systems, but its inhibitory effect on lipid oxidation is limited. Studies have shown that plant polyphenol procyanidins can act as effective antioxidants, but cannot effectively act on lipid surfaces due to their easy degradation. Studies have shown that proanthocyanidins have a high affinity for various types of proteins, so this study prepared a colloidal complex using pea protein (PP) and grape seed proanthocyanidin (GSP) as raw materials, and investigated its formation and stability. The ability of oil-in-water emulsions.
Compounds were prepared by adding different concentrations of GSP solutions to the purified PP solution, and the average particle size, polydispersity index (PDI) and surface potential of the PP-GSP composites were determined by Zetasizer Nano ZS; The pH, temperature and ionic strength of the solution were adjusted to simulate different food system environments to determine the pH stability, thermal stability and salt stability of the complex; by recording the PP-GSP solution in an isothermal titration calorimeter reaction cell The enthalpy change upon binding was analyzed, and a commercial molecular docking program was used to analyze the interaction between the two. Finally, using linseed oil as the research object, the oil-in-water emulsion prepared with this complex as emulsifier was investigated. The functional properties include mean particle size and distribution (laser diffraction), ζ-potential (particle electrophoresis), L*a*b* (colorimeter) and microstructure (laser scanning confocal microscopy).
After the addition of GSP, the isoelectric point of PP decreased, and the thermal stability and salt stability of the composite also decreased. Isothermal titration calorimetry indicated that the interaction between PP and GSP was spontaneous and driven by an enthalpy change, with an estimated stoichiometric number of 3.67 for the interaction, indicating that PP has approximately four sites that can bind to GSP. point. Molecular docking simulation results showed that GSP was tightly bound to PP mainly through hydrogen bond interactions, and more than 20 amino acids were involved, and hydrophobic interactions also played a role.
The average particle size of the emulsion increases with the increase of GSP concentration, indicating that GSP can inhibit particle aggregation during the homogenization process; PP and PP-GSP emulsion droplets have smaller particle size and higher particle size during storage. Surface potential, but the particle size of PP-coated droplets increased significantly after 21 days of storage, while the particle size of PP-GSP-coated droplets remained low throughout the storage process, indicating that PP-GSP complexes were more difficult to make. Droplets aggregated during storage. The results of confocal scanning microscopy also showed that both PP and PP-GSP emulsions contained small oil droplets, and no obvious aggregation occurred, that is, the addition of GSP did not cause the PP-coated oil droplets to coagulate. GSP had little effect on the pH and salt stability of the emulsion, but reduced its thermal stability.
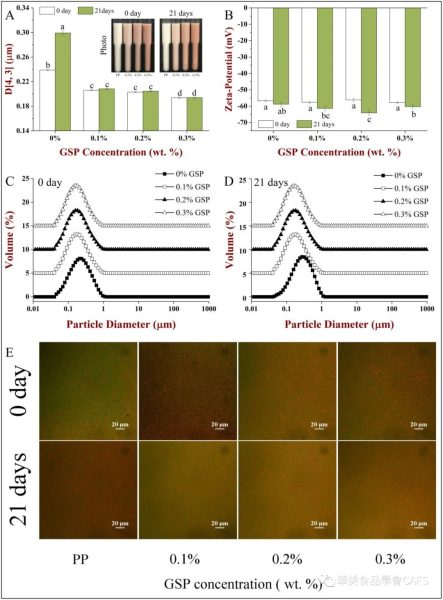
Experiments show that PP and GSP can spontaneously combine into colloidal particles in aqueous solution through hydrogen bonding and hydrophobic interactions. The colloidal particles are relatively small, have a high negative charge, and are resistant to settling. Environmental stability tests showed that GSP changed the isoelectric point of PP and reduced thermal and salt stability. In emulsions, GSP has little effect on the pH and salt stability of the emulsion, but reduces its thermal stability. Conversely, GSP can improve the storage stability of the emulsion. It is worth noting that when GSP is added to the emulsion, it has a distinct orange-red appearance and can be used for some specific applications. Overall, the results of this study are of great significance for the design and development of effective proanthocyanidin release systems, as well as the development of plant-based food emulsions with better physicochemical properties and stability.
