In recent years, as consumer awareness of plant-based diets has grown, the food industry has shown great interest in pea protein because of its sustainability, hypoallergenic properties, and economical benefits. Protein derived from peas, pea protein isolate (PPI), can not only be eaten directly as a protein supplement, but also developed as a functional ingredient, encouraging many recent studies focused on improving the technical functionality of PPIs , including solubility, emulsifying, gelling and foaming ability.
High pressure homogenization (HPH) is a physical technique that can alter the structure of proteins. It applies high pressure, forcing the protein suspension through narrow channels at high speed, during which the structure of the protein is altered by cavitation, compression and shear forces. This technique has been applied to modify the structure of soy, fava and clam proteins in order to alter their technical function. In a recent study, pea protein was treated with industrial-scale microfluidization to improve its solubility. HPH has also been reported to improve the packaging properties of protein-based edible films. For example, it has been reported that high-pressure treatment of soybean protein at 207 MPa for 0 to 30 times can improve the mechanical properties of the film and reduce the water absorption. However, how HPH affects the properties of PPI films is unclear.
Therefore, it is hypothesized that the modification of the PPI structure by HPH can improve the performance of PPI-based films. To verify this, Jingjing Cheng, Leqi Cui*, et al. from the University of Florida, USA, proposed in this study to first describe the structural modification of PPI by HPH, and secondly investigate the color, morphology, mechanical, and moisture resistance of the resulting PPI films. The results of this study will allow us to better understand how HPH affects the performance of PPI edible films.
Structural modification of PPI by HPH
Effect of HPH on PPI Particle Size
The effect of HPH treatment on pea protein structure was first characterized by particle size distribution (Fig. 1A), polydispersity index (PDI), and Z-average hydrodynamic diameter (Fig. 1B). As can be seen in Figure 1A, the PPI of the control group has a unimodal distribution with a single peak at 190 nm. The PPIs treated at 60, 120 and 180 MPa all showed a bimodal distribution, with both HM60 and HM120 showing a new peak at 40 nm, while HM180 showed a new peak at 31 nm. As the pressure was further increased to 240 MPa, the particle size distribution changed back to unimodal, but with a broader shape covering more small-sized particles. The main peak around 190 nm was greatly reduced for all HPH-treated PPIs. These all suggest that the original particles were broken down during the HPH process, resulting in smaller particles. The change in particle size distribution correlates well with PDI (Fig. 1B), which is an indicator of the uniformity of the mean particle size distribution. Untreated PPI had lower PDI, which is consistent with its unimodal distribution. Although no differences were found at different pressures, all HPH-treated PPIs had larger PDI values, which corresponded to a second peak or a wider size distribution shape.
Figure 1B also shows that the particle size of PPI was significantly reduced by HPH treatment, from 171 nm in the control group to 147 nm and 148 nm in HM120 and HM240. In a recent study, the albumin fraction of pea protein was extracted and subjected to 3 microfluidization treatments at pressures ranging from 70 to 130 MPa, and the particle size was found to decrease from 352 nm to 125 nm. However, in this study, pea albumin was aggregated by a pretreatment heated at 90 °C for 1 h. In another study, the particle size of soybean protein isolate was reduced to around 135.8 nm by 30 treatments of HPH at 207 MPa. The particle size reduction of proteins may be due to cavitation, friction and shear forces generated during the high temperature and high pressure process. A smaller particle size indicates that particles with a larger surface area are produced. This change in particle size reduction was reported to be associated with an increase in the foaming ability and gelling properties of the protein. These findings suggest that this structural change may also affect the film-forming ability of pea proteins.
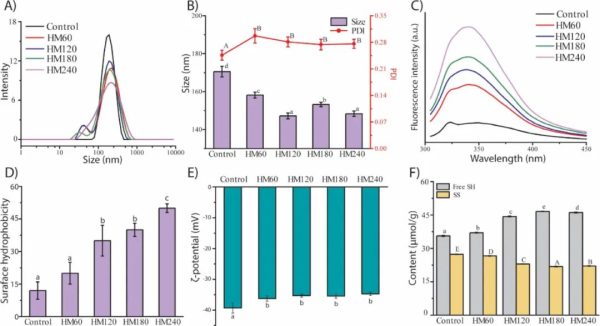
Effects of HPH on PPI unfolding
The unfolding of PPI after HPH treatment was assessed by measuring its intrinsic fluorescence emission spectrum, which is related to the location and tertiary structure of natural chromophores such as Trp, Tyr, and Phe. Typically, when the wavelength of maximum fluorescence intensity (λmax) is greater than 330 nm, the chromophore is considered to be located in a more polar environment; conversely, λmax below 330 nm indicates that the chromophore is located in a more nonpolar environment environment of. As shown in Figure 1C, the untreated PPI exhibited λmax at 323 nm, but a red shift was observed with increasing pressure applied on the PPI (347, 345, 345 and 345 nm), indicating a more polar environment around the chromophore, namely water. Furthermore, the fluorescence intensity increased significantly with increasing treatment pressure. In this study, the red shift of λmax and the increase in fluorescence intensity indicated that after HPH treatment at pressures of 60-240 MPa, the hydrophobic amino acids originally buried inside the protein structure were exposed on the surface of PPI close to water, indicating that the structure has been Expand.
As the unfolding of PPI is confirmed, its surface properties and functional groups are expected to be altered accordingly. To confirm this, the surface hydrophobicity and zeta potential of PPI were further investigated. Surface hydrophobicity (H0) is an index to measure the number of hydrophobic groups on the surface of protein particles. The higher the H0 value, the more hydrophobic groups are distributed along the surface. It was found that, in addition to HM60, HPH led to an increase in H0 (Fig. 1D). For HM60, although the PPI was unfolded after high pressure treatment at 60 MPa (Fig. 1C), this unfolding was not to the extent that the surface hydrophobicity could be altered. For other treatments, the results were consistent with protein unfolding, which allowed more hydrophobic groups to move to the surface under high pressure.
The zeta potential is related to the net surface charge of the protein. The effect of HPH treatment on PPI surface charge is shown in Figure 1E. HPH treatment resulted in a significant decrease in the absolute zeta potential of PPI, with no significant difference between the different treatment groups. This reduction in negative charge may be due to the rearrangement of amino acids on the PPI surface after unfolding. Furthermore, the net surface charge is an indicator of the dispersed or aggregated state of the protein particles. The decrease in absolute zeta potential suggests that HPH-treated PPI favors aggregation. It should be noted that this indication is not contradictory to their reduced particle size; instead, HPH reduced the particle size of pea proteins (Fig. 1B), and these smaller particles, compared with larger particles of untreated PPI, Shows a higher agglomeration trend. Collectively, Figures 1A-1E show that HPH treatment disrupts the original particle of PPI, unfolds PPI, and weakens electrostatic repulsion, while promoting protein-protein intermolecular interactions. This structural modification of PPI by HPH is promising in the application of forming edible films based on PPI.
Other functional groups that may be affected by HPH after protein unfolding include sulfhydryl (SH) and disulfide bonds (SS), although results on the effect of HPH are inconsistent as both SH increases and decreases have been reported. In the current study, it was observed that the free SH groups of PPIs increased significantly after HPH treatment, while the number of SS decreased (Fig. 1F). The decrease in SS may be due to the strong force exerted by HPH to break the SS linkages between different amino acid chains of PPI. This is most likely due to the fact that approximately 32% to 44% of the legumes in pea proteins contain SS bonds from Cys and Met, linking their acidic and basic subunits. The reduction in SS bonds also coincides with the reduction in particle size of PPI (Fig. 1A, 1B), as the natural subunits of soy protein hexamers will thus dissociate with the cleavage of SS bonds. At the same time, the cleavage of the SS bond may also lead to an increase in the free SH content. Additionally, since most SH groups, in their native form, are thought to be hidden in the internal hydrophobic core of the protein, and thus appear in a non-radical form, more free SH may be due to increased exposure to SH after the protein unfolds (Figure 1C). Therefore, the data on SH/SS content supports the unfolding of PPI by HPH treatment, and more importantly, it also suggests that HPH-treated PPI may have better film-forming properties because when the protein matrix is formed during drying , there is more free SH available for potential SS bond formation.
In this study, HPH was applied to modify the structure of PPI, and this structural modification was successfully exploited to form edible films with improved properties. The edible film formed by HPH-treated PPI has better transparency and more uniform surface. Mechanical properties measured by TS and EAB were significantly enhanced due to increased hydrogen bonding and disulfide linkages between polypeptide chains, increased hydrogen bonding between PPI and glycerol, and possibly reduced electrostatic repulsion. PCA successfully separated the samples based on the effect of HPH treatment, and also confirmed the correlation between PPI structural features and film properties. This research is expected to facilitate future research and development of protein films to address plastic contamination of food packaging materials.
